Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
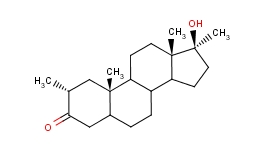
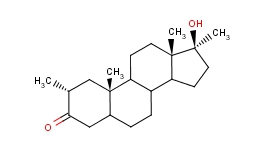
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one] is an AAS, very similar to dihydrotestosterone (DHT), from which it almost completely copies the structure, except for the addition of a methyl group in C1 (similar to primobolan), which to some extent increases its oral bioavailability without causing liver stress. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissue; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one] is an AAS, very similar to dihydrotestosterone (DHT), from which it almost completely copies the structure, except for the addition of a methyl group in C1 (similar to primobolan), which to some extent increases its oral bioavailability without causing liver stress. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissue; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
THE USE OF PROVIRON WITH THE ATHLET FOR FEMALE SEX WAS COMPLETELY: much more anti-estrogenic results in women can be obtained with the help of modern anti-aromatase and for direct action on androgen receptors of fat, especially the specified OSTARIN (with almost zero androgenic effect) or lipolytic action at the level even deeper androgen receptors can be obtained with ANDARINA. Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild antiestrogenic effects. Another use that has made its way to Proviron over the past decade is for its strong ability to bind to SHBG, thereby “releasing” other bound AAS from the binding protein. Unfortunately, however, this action causes a rebound in terms of increasing SHBG, partially mitigated by the usual anti-estrogenic action: in combination with examestane or with other AAS capable of markedly reducing circulating SHBG (especially oral turinabol, winstrol and oxandrolone), it has a very topical action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one] is very similar to AAS to dihydrotestosterone (DHT), the structure of which it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which somewhat increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one] is very similar to AAS to dihydrotestosterone (DHT), the structure of which it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which somewhat increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
THE USE OF PROVIRON WITH WOMEN’S ATHLETES IS FULLY EXCELLENT: much more anti-estrogenic results in women can be obtained with modern anti-aromatase and for direct action on androgen receptors of fat, OSTARINA is especially indicated (with practically zero androgenic effect), or even deeper lipolytic action at the level androgen receptor is achieved using ANDARINA. Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild anti-estrogenic effects. Another use that has made its way to Proviron over the past decade is its strong ability to bind to SHBG, thereby “freeing” other associated AAS from binding protein. Unfortunately, however, this action causes a rebound in terms of an increase in SHBG, partially mitigated by the usual antiestrogenic action: in combination with Examestan or with other AAS capable of markedly reducing circulating SHBG (especially oral administration of turinabol, winstrol and oxandrolone), it has a very actual action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is an AAS, very similar to dihydrotestosterone (DHT), from of which it almost completely copies the structure, except for the addition of a methyl group in C1 (similar to primobolan https://steroidsbuyonline.com/store/oral-steroids/methenolon-primobolan/ ), which to some extent increases its oral bioavailability without causing liver stress. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone… However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissue; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is an AAS, very similar to dihydrotestosterone (DHT), from of which it almost completely copies the structure, except for the addition of a methyl group in C1 (similar to primobolan https://steroidsbuyonline.com/store/oral-steroids/methenolon-primobolan/ ), which to some extent increases its oral bioavailability without causing liver stress. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone… However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissue; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
THE USE OF PROVIRON WITH THE ATHLET FOR FEMALE SEX WAS COMPLETELY: much more anti-estrogenic results in women can be obtained with the help of modern anti-aromatase and for direct action on androgen receptors of fat, especially the specified OSTARIN (with almost zero androgenic effect) or lipolytic action at the level even deeper androgen receptors can be obtained with ANDARINA. Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild antiestrogenic effects… Another use that has made its way to Proviron over the past decade is for its strong ability to bind to SHBG, thereby “releasing” other bound AAS from the binding protein. Unfortunately, however, this action causes a rebound in terms of increasing SHBG, partially mitigated by the usual anti-estrogenic action: in combination with examestane or with other AAS capable of markedly reducing circulating SHBG (especially oral turinabol, winstrol and oxandrolone), it has a very topical action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to the AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is necessary for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues), by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alpha-androstano-3a, 17b-diol… This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to the AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is necessary for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues), by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alpha-androstano-3a, 17b-diol… This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
THE USE OF PROVIRON WITH WOMEN’S ATHLETES IS FULLY EXCELLENT: much more anti-estrogenic results in women can be obtained with modern anti-aromatase and for direct action on androgen receptors of fat, OSTARINA is especially indicated (with practically zero androgenic effect), or even deeper lipolytic action at the level androgen receptor is achieved using ANDARINA. Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild anti-estrogenic effects. Another use that has made its way to Proviron over the past decade is its strong ability to bind to SHBG, thereby “freeing” other associated AAS from binding protein. Unfortunately, however, this action causes a rebound in terms of an increase in SHBG, partially mitigated by the usual antiestrogenic action: in combination with Examestan or with other AAS capable of markedly reducing circulating SHBG (especially oral administration of turinabol, winstrol and oxandrolone), it has a very actual action to “release” associated AAS SHGs.
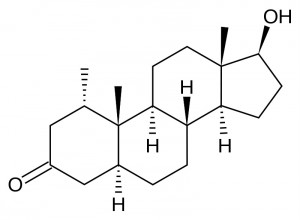
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, is not aromatized. Indeed, it possesses a certain antiestrogenic activity, similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for androgen fat receptors (which stimulate liposis), has long made it typical of pre-competitive… The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, is not aromatized. Indeed, it possesses a certain antiestrogenic activity, similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for androgen fat receptors (which stimulate liposis), has long made it typical of pre-competitive… The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
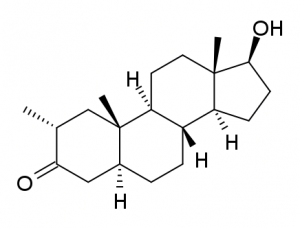
 If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol. The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic… Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-methasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the least “hormonal” sides existing at the time. Its ability (characteristic of more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone, from which it is structurally derived, has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong Affinity for adipose tissue AR (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol. The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic… Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-methasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the least “hormonal” sides existing at the time. Its ability (characteristic of more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone, from which it is structurally derived, has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong Affinity for adipose tissue AR (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
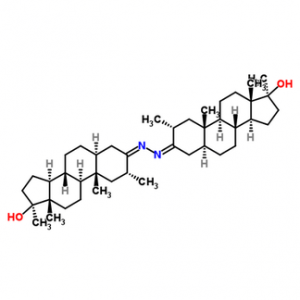 While looking for legal loopholes in the US to continue to commercialize Superdrol, similar molecules have emerged, with perhaps the most interesting being DYMETHAZINE [ 17b-hydroxy-2a, 17b-dimethyl-5a-androstane-3-one-azine ], sold under various names such as HALO SUSPENSION, DIMETHADROL, DMZ, PRO-TRENAZINE, etc. This product is nothing more than two Superdrol molecules attacked by a single nitrogen atom: quite surprising what it is the same potent and bioavailable compound compared to Superdrol, but with much less toxicity to the liver; it may be metabolized similarly to oxandrolone, recent studies of which approximate its toxicity level: when administered over 45 days at a dose of 0.25 mg per 1 kg of DMZ, no liver toxicity was detected in 50% of subjects, and insignificant in the remaining 50%. However, DMZ has a certain specificity for Superdrol, as it has an androgen / anabolic ratio of 6/330 when administered orally (two molecules are broken down orally), whereas when injected, the ratio is 26/800. !!. Otherwise, DMZ exhibits the same receptor affinity and the same Superdrol non-aromatization. Even the DMZ does not represent a truly new DS: in fact, this product was first synthesized by Italian researchers in 1960..
While looking for legal loopholes in the US to continue to commercialize Superdrol, similar molecules have emerged, with perhaps the most interesting being DYMETHAZINE [ 17b-hydroxy-2a, 17b-dimethyl-5a-androstane-3-one-azine ], sold under various names such as HALO SUSPENSION, DIMETHADROL, DMZ, PRO-TRENAZINE, etc. This product is nothing more than two Superdrol molecules attacked by a single nitrogen atom: quite surprising what it is the same potent and bioavailable compound compared to Superdrol, but with much less toxicity to the liver; it may be metabolized similarly to oxandrolone, recent studies of which approximate its toxicity level: when administered over 45 days at a dose of 0.25 mg per 1 kg of DMZ, no liver toxicity was detected in 50% of subjects, and insignificant in the remaining 50%. However, DMZ has a certain specificity for Superdrol, as it has an androgen / anabolic ratio of 6/330 when administered orally (two molecules are broken down orally), whereas when injected, the ratio is 26/800. !!. Otherwise, DMZ exhibits the same receptor affinity and the same Superdrol non-aromatization. Even the DMZ does not represent a truly new DS: in fact, this product was first synthesized by Italian researchers in 1960..
CYANOSTANE, also called CYNABOL 10 mg [ 2-cyano-17a-methyl-17b-hydroxy-androst-3-one ], is a developer based on the SUPERDROL structure, from which it differs only in replacement The 2-alpha methyl bond (which helps stabilize Superdrol’s 3-ket group, making it more anabolic) to the blue bond. The methyl bond at C-17 is retained and hence the hepatotoxicity of the product. The effect of using hydrogen cyanide seems unattractive, slightly androgenic and strongly AR like SUPERDROL, but about twice as powerful as Superdrol: 45/800 androgenic / anabolic strength.
Also the effect on lipid is less negative than Superdrol. However, it should be borne in mind that studies done on the other two 2-cyanosteroids (2-cyano-DHT and 2-cyano-progesterone) would show strong adrenal inhibition, which would complicate the situation, on the one hand, the level of cortisol is probably lower (perhaps too much), but also lethargy. It is not known whether this is a peculiar characteristic of the two studied 2-cyanosteroids or is common for all steroids containing modifications of this type.
 If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, with the exception of the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver… However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success, being particularly mediated estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, with the exception of the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver… However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success, being particularly mediated estrogen in both the female and male brains.
THE USE OF PROVIRON WITH WOMEN’S ATHLETES IS FULLY EXCELLENT: Much more anti-estrogenic results in women can be obtained with modern anti-aromatase and for direct action on androgen receptors of fat, OSTARINA is especially indicated (with practically zero androgenic effect), or an even deeper lipolytic effect on androgen receptor level achieved with ANDARINA… Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild anti-estrogenic effects. Another use that has made its way to Proviron over the past decade is its strong ability to bind to SHBG, thereby “freeing” other associated AAS from binding protein. Unfortunately, however, this action causes a rebound in terms of an increase in SHBG, partially mitigated by the usual antiestrogenic action: in combination with Examestan or with other AAS capable of markedly reducing circulating SHBG (especially oral administration of turinabol, winstrol and oxandrolone), it has a very actual action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, no aromatization… Indeed, it possesses a certain antiestrogenic activity similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for fatty androgen receptors (which stimulate liposis), has long made it typical of pre-competition. The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, no aromatization… Indeed, it possesses a certain antiestrogenic activity similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for fatty androgen receptors (which stimulate liposis), has long made it typical of pre-competition. The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
 If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol… The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. a keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic. Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-metaasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the fewest “hormonal” sides existing at the time. Its ability (common to more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone from which it is structurally derived, it has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong affinity for AR adipose tissue (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol… The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. a keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic. Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-metaasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the fewest “hormonal” sides existing at the time. Its ability (common to more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone from which it is structurally derived, it has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong affinity for AR adipose tissue (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
 If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is an AAS, very similar to dihydrotestosterone (DHT), from of which it almost completely copies the structure, except for the addition of a methyl group in C1 (similar to primobolan), which to some extent increases its oral bioavailability without causing liver stress. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone… However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissue; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is an AAS, very similar to dihydrotestosterone (DHT), from of which it almost completely copies the structure, except for the addition of a methyl group in C1 (similar to primobolan), which to some extent increases its oral bioavailability without causing liver stress. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone… However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissue; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
THE USE OF PROVIRON WITH THE ATHLET FOR FEMALE SEX WAS COMPLETELY: much more anti-estrogenic results in women can be obtained with the help of modern anti-aromatase and for direct action on androgen receptors of fat, especially the specified OSTARIN (with almost zero androgenic effect) or lipolytic action at the level even deeper androgen receptors can be obtained with ANDARINA. Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild antiestrogenic effects… Another use that has made its way to Proviron over the past decade is for its strong ability to bind to SHBG, thereby “releasing” other bound AAS from the binding protein. Unfortunately, however, this action causes a rebound in terms of increasing SHBG, partially mitigated by the usual anti-estrogenic action: in combination with examestane or with other AAS capable of markedly reducing circulating SHBG (especially oral turinabol, winstrol and oxandrolone), it has a very topical action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to the AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is necessary for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues), by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alpha-androstano-3a, 17b-diol… This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to the AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is necessary for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues), by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alpha-androstano-3a, 17b-diol… This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
THE USE OF PROVIRON WITH WOMEN’S ATHLETES IS FULLY EXCELLENT: much more anti-estrogenic results in women can be obtained with modern anti-aromatase and for direct action on androgen receptors of fat, OSTARINA is especially indicated (with practically zero androgenic effect), or even deeper lipolytic action at the level androgen receptor is achieved using ANDARINA. Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild anti-estrogenic effects. Another use that has made its way to Proviron over the past decade is its strong ability to bind to SHBG, thereby “freeing” other associated AAS from binding protein. Unfortunately, however, this action causes a rebound in terms of an increase in SHBG, partially mitigated by the usual antiestrogenic action: in combination with Examestan or with other AAS capable of markedly reducing circulating SHBG (especially oral administration of turinabol, winstrol and oxandrolone), it has a very actual action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, is not aromatized. Indeed, it possesses a certain antiestrogenic activity, similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for androgen fat receptors (which stimulate liposis), has long made it typical of pre-competitive… The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, is not aromatized. Indeed, it possesses a certain antiestrogenic activity, similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for androgen fat receptors (which stimulate liposis), has long made it typical of pre-competitive… The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
 If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol. The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic… Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-methasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the least “hormonal” sides existing at the time. Its ability (characteristic of more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone, from which it is structurally derived, has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong Affinity for adipose tissue AR (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol. The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic… Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-methasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the least “hormonal” sides existing at the time. Its ability (characteristic of more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone, from which it is structurally derived, has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong Affinity for adipose tissue AR (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
 While looking for legal loopholes in the US to continue to commercialize Superdrol, similar molecules have emerged, with perhaps the most interesting being DYMETHAZINE [ 17b-hydroxy-2a, 17b-dimethyl-5a-androstane-3-one-azine ], sold under various names such as HALO SUSPENSION, DIMETHADROL, DMZ, PRO-TRENAZINE, etc. This product is nothing more than two Superdrol molecules attacked by a single nitrogen atom: quite surprising what it is the same potent and bioavailable compound compared to Superdrol, but with much less toxicity to the liver; it may be metabolized similarly to oxandrolone, recent studies of which approximate its toxicity level: when administered over 45 days at a dose of 0.25 mg per 1 kg of DMZ, no liver toxicity was detected in 50% of subjects, and insignificant in the remaining 50%. However, DMZ has a certain specificity for Superdrol, as it has an androgen / anabolic ratio of 6/330 when administered orally (two molecules are broken down orally), whereas when injected, the ratio is 26/800. !!. Otherwise, DMZ exhibits the same receptor affinity and the same Superdrol non-aromatization. Even the DMZ does not represent a truly new DS: in fact, this product was first synthesized by Italian researchers in 1960..
While looking for legal loopholes in the US to continue to commercialize Superdrol, similar molecules have emerged, with perhaps the most interesting being DYMETHAZINE [ 17b-hydroxy-2a, 17b-dimethyl-5a-androstane-3-one-azine ], sold under various names such as HALO SUSPENSION, DIMETHADROL, DMZ, PRO-TRENAZINE, etc. This product is nothing more than two Superdrol molecules attacked by a single nitrogen atom: quite surprising what it is the same potent and bioavailable compound compared to Superdrol, but with much less toxicity to the liver; it may be metabolized similarly to oxandrolone, recent studies of which approximate its toxicity level: when administered over 45 days at a dose of 0.25 mg per 1 kg of DMZ, no liver toxicity was detected in 50% of subjects, and insignificant in the remaining 50%. However, DMZ has a certain specificity for Superdrol, as it has an androgen / anabolic ratio of 6/330 when administered orally (two molecules are broken down orally), whereas when injected, the ratio is 26/800. !!. Otherwise, DMZ exhibits the same receptor affinity and the same Superdrol non-aromatization. Even the DMZ does not represent a truly new DS: in fact, this product was first synthesized by Italian researchers in 1960..
CYANOSTANE, also called CYNABOL 10 mg [ 2-cyano-17a-methyl-17b-hydroxy-androst-3-one ], is a developer based on the SUPERDROL structure, from which it differs only in replacement The 2-alpha methyl bond (which helps stabilize Superdrol’s 3-ket group, making it more anabolic) to the blue bond. The methyl bond at C-17 is retained and hence the hepatotoxicity of the product. The effect of using hydrogen cyanide seems unattractive, slightly androgenic and strongly AR like SUPERDROL, but about twice as powerful as Superdrol: 45/800 androgenic / anabolic strength.
Also the effect on lipid is less negative than Superdrol. However, it should be borne in mind that studies done on the other two 2-cyanosteroids (2-cyano-DHT and 2-cyano-progesterone) would show strong adrenal inhibition, which would complicate the situation, on the one hand, the level of cortisol is probably lower (perhaps too much), but also lethargy. It is not known whether this is a peculiar characteristic of the two studied 2-cyanosteroids or is common for all steroids containing modifications of this type.
 If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, with the exception of the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver… However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success, being particularly mediated estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, with the exception of the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver… However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success, being particularly mediated estrogen in both the female and male brains.
THE USE OF PROVIRON WITH WOMEN’S ATHLETES IS FULLY EXCELLENT: Much more anti-estrogenic results in women can be obtained with modern anti-aromatase and for direct action on androgen receptors of fat, OSTARINA is especially indicated (with practically zero androgenic effect), or an even deeper lipolytic effect on androgen receptor level achieved with ANDARINA… Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild anti-estrogenic effects. Another use that has made its way to Proviron over the past decade is its strong ability to bind to SHBG, thereby “freeing” other associated AAS from binding protein. Unfortunately, however, this action causes a rebound in terms of an increase in SHBG, partially mitigated by the usual antiestrogenic action: in combination with Examestan or with other AAS capable of markedly reducing circulating SHBG (especially oral administration of turinabol, winstrol and oxandrolone), it has a very actual action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, no aromatization… Indeed, it possesses a certain antiestrogenic activity similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for fatty androgen receptors (which stimulate liposis), has long made it typical of pre-competition. The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, no aromatization… Indeed, it possesses a certain antiestrogenic activity similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for fatty androgen receptors (which stimulate liposis), has long made it typical of pre-competition. The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
 If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol… The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. a keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic. Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-metaasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the fewest “hormonal” sides existing at the time. Its ability (common to more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone from which it is structurally derived, it has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong affinity for AR adipose tissue (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol… The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. a keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic. Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-metaasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the fewest “hormonal” sides existing at the time. Its ability (common to more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone from which it is structurally derived, it has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong affinity for AR adipose tissue (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
 While looking for legal loopholes in the US to continue to commercialize Superdrol, similar molecules have emerged, with perhaps the most interesting being DYMETHAZINE [ 17b-hydroxy-2a, 17b-dimethyl-5a-androstane-3-one-azine ], sold under various names such as HALO SUSPENSION, DIMETHADROL, DMZ, PRO-TRENAZINE, etc. This product is nothing more than two Superdrol molecules attacked by a single nitrogen atom: quite surprising what it is the same potent and bioavailable compound compared to Superdrol, but with much less toxicity to the liver; it may be metabolized similarly to oxandrolone, recent studies of which approximate its toxicity level: when administered over 45 days at a dose of 0.25 mg per 1 kg of DMZ, no liver toxicity was detected in 50% of subjects, and insignificant in the remaining 50%. However, DMZ has a certain specificity for Superdrol, as it has an androgen / anabolic ratio of 6/330 when administered orally (two molecules are broken down orally), whereas when injected, the ratio is 26/800. !!. Otherwise, DMZ exhibits the same receptor affinity and the same Superdrol non-aromatization. Even the DMZ does not represent a truly new DS: in fact, this product was first synthesized by Italian researchers in 1960.
While looking for legal loopholes in the US to continue to commercialize Superdrol, similar molecules have emerged, with perhaps the most interesting being DYMETHAZINE [ 17b-hydroxy-2a, 17b-dimethyl-5a-androstane-3-one-azine ], sold under various names such as HALO SUSPENSION, DIMETHADROL, DMZ, PRO-TRENAZINE, etc. This product is nothing more than two Superdrol molecules attacked by a single nitrogen atom: quite surprising what it is the same potent and bioavailable compound compared to Superdrol, but with much less toxicity to the liver; it may be metabolized similarly to oxandrolone, recent studies of which approximate its toxicity level: when administered over 45 days at a dose of 0.25 mg per 1 kg of DMZ, no liver toxicity was detected in 50% of subjects, and insignificant in the remaining 50%. However, DMZ has a certain specificity for Superdrol, as it has an androgen / anabolic ratio of 6/330 when administered orally (two molecules are broken down orally), whereas when injected, the ratio is 26/800. !!. Otherwise, DMZ exhibits the same receptor affinity and the same Superdrol non-aromatization. Even the DMZ does not represent a truly new DS: in fact, this product was first synthesized by Italian researchers in 1960.
CYANOSTANE, also called CYNABOL 10 mg [ 2-cyano-17a-methyl-17b-hydroxy-androst-3-one], is a developer based on the SUPERDROL structure, from which it differs only by replacing the 2-alpha-methyl bond (which helps stabilize Superdrol’s 3-ket group, making it more anabolic) with a cyanide bond. The methyl bond at C-17 is retained and hence the hepatotoxicity of the product. The effect of using hydrogen cyanide seems unattractive, slightly androgenic and strongly AR like SUPERDROL, but about twice as powerful as Superdrol: 45/800 androgenic / anabolic strength.
Also the effect on lipid is less negative than Superdrol. However, it should be borne in mind that studies done on the other two 2-cyanosteroids (2-cyano-DHT and 2-cyano-progesterone) would show strong adrenal inhibition, which would complicate the situation, on the one hand, the level of cortisol is probably lower (perhaps too much), but also lethargy. It is not known whether this is a peculiar characteristic of the two studied 2-cyanosteroids or is common for all steroids containing modifications of this type.
 If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one] is very similar to AAS to dihydrotestosterone (DHT), the structure of which it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which somewhat increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success, being particularly mediated estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one] is very similar to AAS to dihydrotestosterone (DHT), the structure of which it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which somewhat increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success, being particularly mediated estrogen in both the female and male brains.
THE USE OF PROVIRON WITH WOMEN’S ATHLETES IS FULLY EXCELLENT: Much more anti-estrogenic results in women can be obtained with modern anti-aromatase and for direct action on androgen receptors of fat, OSTARINA is especially indicated (with practically zero androgenic effect), or an even deeper lipolytic effect on the level of androgen receptors is achieved with the help of ANDARINA. Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild anti-estrogenic effects. Another use that has made its way to Proviron over the past decade is its strong ability to bind to SHBG, thereby “freeing” other associated AAS from binding protein. Unfortunately, however, this action causes a rebound in terms of an increase in SHBG, partially mitigated by the usual antiestrogenic action: in combination with Examestan or with other AAS capable of markedly reducing circulating SHBG (especially oral administration of turinabol, winstrol and oxandrolone), it has a very actual action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is an AAS, very similar to dihydrotestosterone (DHT), from of which it almost completely copies the structure, except for the addition of a methyl group in C1 (similar to primobolan https://steroidsbuyonline.com/store/oral-steroids/methenolon-primobolan/ ), which to some extent increases its oral bioavailability without causing liver stress. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone… However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissue; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is an AAS, very similar to dihydrotestosterone (DHT), from of which it almost completely copies the structure, except for the addition of a methyl group in C1 (similar to primobolan https://steroidsbuyonline.com/store/oral-steroids/methenolon-primobolan/ ), which to some extent increases its oral bioavailability without causing liver stress. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone… However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissue; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
THE USE OF PROVIRON WITH THE ATHLET FOR FEMALE SEX WAS COMPLETELY: much more anti-estrogenic results in women can be obtained with the help of modern anti-aromatase and for direct action on androgen receptors of fat, especially the specified OSTARIN (with almost zero androgenic effect) or lipolytic action at the level even deeper androgen receptors can be obtained with ANDARINA. Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild antiestrogenic effects… Another use that has made its way to Proviron over the past decade is for its strong ability to bind to SHBG, thereby “releasing” other bound AAS from the binding protein. Unfortunately, however, this action causes a rebound in terms of increasing SHBG, partially mitigated by the usual anti-estrogenic action: in combination with examestane or with other AAS capable of markedly reducing circulating SHBG (especially oral turinabol, winstrol and oxandrolone), it has a very topical action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to the AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is necessary for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues), by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alpha-androstano-3a, 17b-diol… This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to the AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is necessary for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues), by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alpha-androstano-3a, 17b-diol… This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
THE USE OF PROVIRON WITH WOMEN’S ATHLETES IS FULLY EXCELLENT: much more anti-estrogenic results in women can be obtained with modern anti-aromatase and for direct action on androgen receptors of fat, OSTARINA is especially indicated (with practically zero androgenic effect), or even deeper lipolytic action at the level androgen receptor is achieved using ANDARINA. Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild anti-estrogenic effects. Another use that has made its way to Proviron over the past decade is its strong ability to bind to SHBG, thereby “freeing” other associated AAS from binding protein. Unfortunately, however, this action causes a rebound in terms of an increase in SHBG, partially mitigated by the usual antiestrogenic action: in combination with Examestan or with other AAS capable of markedly reducing circulating SHBG (especially oral administration of turinabol, winstrol and oxandrolone), it has a very actual action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, is not aromatized. Indeed, it possesses a certain antiestrogenic activity, similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for androgen fat receptors (which stimulate liposis), has long made it typical of pre-competitive… The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, is not aromatized. Indeed, it possesses a certain antiestrogenic activity, similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for androgen fat receptors (which stimulate liposis), has long made it typical of pre-competitive… The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
 If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol. The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic… Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-methasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the least “hormonal” sides existing at the time. Its ability (characteristic of more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone, from which it is structurally derived, has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong Affinity for adipose tissue AR (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol. The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic… Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-methasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the least “hormonal” sides existing at the time. Its ability (characteristic of more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone, from which it is structurally derived, has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong Affinity for adipose tissue AR (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
 While looking for legal loopholes in the US to continue to commercialize Superdrol, similar molecules have emerged, with perhaps the most interesting being DYMETHAZINE [ 17b-hydroxy-2a, 17b-dimethyl-5a-androstane-3-one-azine ], sold under various names such as HALO SUSPENSION, DIMETHADROL, DMZ, PRO-TRENAZINE, etc. This product is nothing more than two Superdrol molecules attacked by a single nitrogen atom: quite surprising what it is the same potent and bioavailable compound compared to Superdrol, but with much less toxicity to the liver; it may be metabolized similarly to oxandrolone, recent studies of which approximate its toxicity level: when administered over 45 days at a dose of 0.25 mg per 1 kg of DMZ, no liver toxicity was detected in 50% of subjects, and insignificant in the remaining 50%. However, DMZ has a certain specificity for Superdrol, as it has an androgen / anabolic ratio of 6/330 when administered orally (two molecules are broken down orally), whereas when injected, the ratio is 26/800. !!. Otherwise, DMZ exhibits the same receptor affinity and the same Superdrol non-aromatization. Even the DMZ does not represent a truly new DS: in fact, this product was first synthesized by Italian researchers in 1960..
While looking for legal loopholes in the US to continue to commercialize Superdrol, similar molecules have emerged, with perhaps the most interesting being DYMETHAZINE [ 17b-hydroxy-2a, 17b-dimethyl-5a-androstane-3-one-azine ], sold under various names such as HALO SUSPENSION, DIMETHADROL, DMZ, PRO-TRENAZINE, etc. This product is nothing more than two Superdrol molecules attacked by a single nitrogen atom: quite surprising what it is the same potent and bioavailable compound compared to Superdrol, but with much less toxicity to the liver; it may be metabolized similarly to oxandrolone, recent studies of which approximate its toxicity level: when administered over 45 days at a dose of 0.25 mg per 1 kg of DMZ, no liver toxicity was detected in 50% of subjects, and insignificant in the remaining 50%. However, DMZ has a certain specificity for Superdrol, as it has an androgen / anabolic ratio of 6/330 when administered orally (two molecules are broken down orally), whereas when injected, the ratio is 26/800. !!. Otherwise, DMZ exhibits the same receptor affinity and the same Superdrol non-aromatization. Even the DMZ does not represent a truly new DS: in fact, this product was first synthesized by Italian researchers in 1960..
CYANOSTANE, also called CYNABOL 10 mg [ 2-cyano-17a-methyl-17b-hydroxy-androst-3-one ], is a developer based on the SUPERDROL structure, from which it differs only in replacement The 2-alpha methyl bond (which helps stabilize Superdrol’s 3-ket group, making it more anabolic) to the blue bond. The methyl bond at C-17 is retained and hence the hepatotoxicity of the product. The effect of using hydrogen cyanide seems unattractive, slightly androgenic and strongly AR like SUPERDROL, but about twice as powerful as Superdrol: 45/800 androgenic / anabolic strength.
Also the effect on lipid is less negative than Superdrol. However, it should be borne in mind that studies done on the other two 2-cyanosteroids (2-cyano-DHT and 2-cyano-progesterone) would show strong adrenal inhibition, which would complicate the situation, on the one hand, the level of cortisol is probably lower (perhaps too much), but also lethargy. It is not known whether this is a peculiar characteristic of the two studied 2-cyanosteroids or is common for all steroids containing modifications of this type.
 If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, with the exception of the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver… However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success, being particularly mediated estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, with the exception of the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver… However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success, being particularly mediated estrogen in both the female and male brains.
THE USE OF PROVIRON WITH WOMEN’S ATHLETES IS FULLY EXCELLENT: Much more anti-estrogenic results in women can be obtained with modern anti-aromatase and for direct action on androgen receptors of fat, OSTARINA is especially indicated (with practically zero androgenic effect), or an even deeper lipolytic effect on androgen receptor level achieved with ANDARINA… Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild anti-estrogenic effects. Another use that has made its way to Proviron over the past decade is its strong ability to bind to SHBG, thereby “freeing” other associated AAS from binding protein. Unfortunately, however, this action causes a rebound in terms of an increase in SHBG, partially mitigated by the usual antiestrogenic action: in combination with Examestan or with other AAS capable of markedly reducing circulating SHBG (especially oral administration of turinabol, winstrol and oxandrolone), it has a very actual action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, no aromatization… Indeed, it possesses a certain antiestrogenic activity similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for fatty androgen receptors (which stimulate liposis), has long made it typical of pre-competition. The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, no aromatization… Indeed, it possesses a certain antiestrogenic activity similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for fatty androgen receptors (which stimulate liposis), has long made it typical of pre-competition. The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
 If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol… The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. a keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic. Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-metaasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the fewest “hormonal” sides existing at the time. Its ability (common to more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone from which it is structurally derived, it has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong affinity for AR adipose tissue (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol… The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. a keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic. Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-metaasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the fewest “hormonal” sides existing at the time. Its ability (common to more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone from which it is structurally derived, it has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong affinity for AR adipose tissue (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
 If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is an AAS, very similar to dihydrotestosterone (DHT), from of which it almost completely copies the structure, except for the addition of a methyl group in C1 (similar to primobolan), which to some extent increases its oral bioavailability without causing liver stress. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone… However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissue; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is an AAS, very similar to dihydrotestosterone (DHT), from of which it almost completely copies the structure, except for the addition of a methyl group in C1 (similar to primobolan), which to some extent increases its oral bioavailability without causing liver stress. However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone… However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissue; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
THE USE OF PROVIRON WITH THE ATHLET FOR FEMALE SEX WAS COMPLETELY: much more anti-estrogenic results in women can be obtained with the help of modern anti-aromatase and for direct action on androgen receptors of fat, especially the specified OSTARIN (with almost zero androgenic effect) or lipolytic action at the level even deeper androgen receptors can be obtained with ANDARINA. Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild antiestrogenic effects… Another use that has made its way to Proviron over the past decade is for its strong ability to bind to SHBG, thereby “releasing” other bound AAS from the binding protein. Unfortunately, however, this action causes a rebound in terms of increasing SHBG, partially mitigated by the usual anti-estrogenic action: in combination with examestane or with other AAS capable of markedly reducing circulating SHBG (especially oral turinabol, winstrol and oxandrolone), it has a very topical action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to the AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is necessary for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues), by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alpha-androstano-3a, 17b-diol… This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to the AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, except for the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver. However, this change does not increase the stability of the C3 keto group, which is necessary for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues), by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alpha-androstano-3a, 17b-diol… This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success as it is especially is mediated by estrogen in both the female and male brains.
THE USE OF PROVIRON WITH WOMEN’S ATHLETES IS FULLY EXCELLENT: much more anti-estrogenic results in women can be obtained with modern anti-aromatase and for direct action on androgen receptors of fat, OSTARINA is especially indicated (with practically zero androgenic effect), or even deeper lipolytic action at the level androgen receptor is achieved using ANDARINA. Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild anti-estrogenic effects. Another use that has made its way to Proviron over the past decade is its strong ability to bind to SHBG, thereby “freeing” other associated AAS from binding protein. Unfortunately, however, this action causes a rebound in terms of an increase in SHBG, partially mitigated by the usual antiestrogenic action: in combination with Examestan or with other AAS capable of markedly reducing circulating SHBG (especially oral administration of turinabol, winstrol and oxandrolone), it has a very actual action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, is not aromatized. Indeed, it possesses a certain antiestrogenic activity, similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for androgen fat receptors (which stimulate liposis), has long made it typical of pre-competitive… The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, is not aromatized. Indeed, it possesses a certain antiestrogenic activity, similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for androgen fat receptors (which stimulate liposis), has long made it typical of pre-competitive… The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
 If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol. The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic… Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-methasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the least “hormonal” sides existing at the time. Its ability (characteristic of more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone, from which it is structurally derived, has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong Affinity for adipose tissue AR (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol. The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic… Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-methasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the least “hormonal” sides existing at the time. Its ability (characteristic of more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone, from which it is structurally derived, has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong Affinity for adipose tissue AR (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
 While looking for legal loopholes in the US to continue to commercialize Superdrol, similar molecules have emerged, with perhaps the most interesting being DYMETHAZINE [ 17b-hydroxy-2a, 17b-dimethyl-5a-androstane-3-one-azine ], sold under various names such as HALO SUSPENSION, DIMETHADROL, DMZ, PRO-TRENAZINE, etc. This product is nothing more than two Superdrol molecules attacked by a single nitrogen atom: quite surprising what it is the same potent and bioavailable compound compared to Superdrol, but with much less toxicity to the liver; it may be metabolized similarly to oxandrolone, recent studies of which approximate its toxicity level: when administered over 45 days at a dose of 0.25 mg per 1 kg of DMZ, no liver toxicity was detected in 50% of subjects, and insignificant in the remaining 50%. However, DMZ has a certain specificity for Superdrol, as it has an androgen / anabolic ratio of 6/330 when administered orally (two molecules are broken down orally), whereas when injected, the ratio is 26/800. !!. Otherwise, DMZ exhibits the same receptor affinity and the same Superdrol non-aromatization. Even the DMZ does not represent a truly new DS: in fact, this product was first synthesized by Italian researchers in 1960..
While looking for legal loopholes in the US to continue to commercialize Superdrol, similar molecules have emerged, with perhaps the most interesting being DYMETHAZINE [ 17b-hydroxy-2a, 17b-dimethyl-5a-androstane-3-one-azine ], sold under various names such as HALO SUSPENSION, DIMETHADROL, DMZ, PRO-TRENAZINE, etc. This product is nothing more than two Superdrol molecules attacked by a single nitrogen atom: quite surprising what it is the same potent and bioavailable compound compared to Superdrol, but with much less toxicity to the liver; it may be metabolized similarly to oxandrolone, recent studies of which approximate its toxicity level: when administered over 45 days at a dose of 0.25 mg per 1 kg of DMZ, no liver toxicity was detected in 50% of subjects, and insignificant in the remaining 50%. However, DMZ has a certain specificity for Superdrol, as it has an androgen / anabolic ratio of 6/330 when administered orally (two molecules are broken down orally), whereas when injected, the ratio is 26/800. !!. Otherwise, DMZ exhibits the same receptor affinity and the same Superdrol non-aromatization. Even the DMZ does not represent a truly new DS: in fact, this product was first synthesized by Italian researchers in 1960..
CYANOSTANE, also called CYNABOL 10 mg [ 2-cyano-17a-methyl-17b-hydroxy-androst-3-one ], is a developer based on the SUPERDROL structure, from which it differs only in replacement The 2-alpha methyl bond (which helps stabilize Superdrol’s 3-ket group, making it more anabolic) to the blue bond. The methyl bond at C-17 is retained and hence the hepatotoxicity of the product. The effect of using hydrogen cyanide seems unattractive, slightly androgenic and strongly AR like SUPERDROL, but about twice as powerful as Superdrol: 45/800 androgenic / anabolic strength.
Also the effect on lipid is less negative than Superdrol. However, it should be borne in mind that studies done on the other two 2-cyanosteroids (2-cyano-DHT and 2-cyano-progesterone) would show strong adrenal inhibition, which would complicate the situation, on the one hand, the level of cortisol is probably lower (perhaps too much), but also lethargy. It is not known whether this is a peculiar characteristic of the two studied 2-cyanosteroids or is common for all steroids containing modifications of this type.
 If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
If we add methylation at position C-17 to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one
Always in order to better understand the ways and activities of various steroid and prohormone developers, we analyze the whole chain of modification of AAS molecules.
 Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, with the exception of the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver… However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success, being particularly mediated estrogen in both the female and male brains.
Mesterolone (PROVIRON) [ 1-alpha-methyl-17-beta-hydroxy-5-alpha-androstan-3-one ] is very similar to AAS to dihydrotestosterone (DHT), whose structure it reproduces almost completely, with the exception of the addition of a methyl group (similar to primobolan) to C1, which to some extent increases its oral bioavailability without causing stress in the liver… However, this change does not increase the stability of the C3 keto group, which is required for anabolic activity at the muscle level: in fact, although, like DHT, it is very similar to AR receptors such as DHT, it is rapidly transformed in muscle tissue (but not in other androgen-responsive tissues) by the enzyme 3alpha-hydroxysteroid reductase (3a-HSD) in an almost inactive metabolite called 5alfa-androstano-3a, 17b-diol. This explains, along with low bioavailability, the low anabolic activity (30 versus 100 testosterone) performed by mesterolone. However, it should be remembered that androgen receptors present in adipocytes exhibit lipolytic activity and that the 3a-HSD enzyme is not present in adipose tissues; therefore Proviron has a decent lipolytic activity, also supported by its antiestrogenic activity (modest compared to modern aromatase inhibitors), mediated by its ability to bind to some extent to the P-450 enzyme required for the aromatic process: for this reason, the reason why Proviron is in the past often used by athletes, but with a clear virilizing effect; in fact, mesterolone, which does not undergo 3-alpha hydroxy reduction in androgen-sensitive tissues, retains an androgenic potency of 150 in relation to testosterone 150. For this reason, it has also been used to restore libido, but with little success, being particularly mediated estrogen in both the female and male brains.
THE USE OF PROVIRON WITH WOMEN’S ATHLETES IS FULLY EXCELLENT: Much more anti-estrogenic results in women can be obtained with modern anti-aromatase and for direct action on androgen receptors of fat, OSTARINA is especially indicated (with practically zero androgenic effect), or an even deeper lipolytic effect on androgen receptor level achieved with ANDARINA… Even a low dose of trenbolone (with a 600/185 ratio and therefore a therapeutic index above 3) will be much more lipolytic than Proviron, not to mention the protective properties of a lean mass.
Although it is in no way transformed into estrogen (which is a reduced 5-alpha molecule), it sends a suppression signal to the HPTA associated with its inherent androgenic action: the modesty of this action (does not matter until 100- 150 mg per day) is likely due to its low bioavailability as well as mild anti-estrogenic effects. Another use that has made its way to Proviron over the past decade is its strong ability to bind to SHBG, thereby “freeing” other associated AAS from binding protein. Unfortunately, however, this action causes a rebound in terms of an increase in SHBG, partially mitigated by the usual antiestrogenic action: in combination with Examestan or with other AAS capable of markedly reducing circulating SHBG (especially oral administration of turinabol, winstrol and oxandrolone), it has a very actual action to “release” associated AAS SHGs.
 If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, no aromatization… Indeed, it possesses a certain antiestrogenic activity similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for fatty androgen receptors (which stimulate liposis), has long made it typical of pre-competition. The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
If we want to get drostanolone (MASTERON) [ 2a-methyl-dihydro-testosterone ] instead of adding the methyl group C-1 to dihydrotestosterone, we add one to C-2, thus getting an increase resistance to the 3a-HSD keto group C-3, but not improving oral bioavailability compared to DHT. Slightly improves biological activity due to slightly lower affinity for SHBG. This type of methylation (i C-2) is completely non-toxic to the liver. Masteron, which is a reduced 5-alpha compound, no aromatization… Indeed, it possesses a certain antiestrogenic activity similar to that of mesterolone, quantified similarly to that of Nolvadex, which, together with its strong affinity for fatty androgen receptors (which stimulate liposis), has long made it typical of pre-competition. The official androgenic / anabolic ratio is: 25/62, which makes it a completely unsuitable product for mass, but also mediocre to define, despite its antiestrogenic properties; in fact, the anabolic strength is too low to preserve muscle mass at low calories.Not surprisingly, with the advent of new anti-aromatases, Masteron is widely phased out in the United States, including for cutting. Until the 1990s, the testosterone-masteron or dianabol-masteron association was widespread enough for a “clean” mass. But already in the context, Masteron was practically useless since the appearance of trenbolone: the latter has even more pronounced lipolytic properties and completely displaces Maston with androgen receptors. Virtually any androgen receptor AAS or PH is capable of displacing Masteron from the androgen receptor; deterioration of the position of the masteron is an almost exclusive sign of affinity (albeit weak) for androgen receptors; Masteron does not bind to any non-androgenic receptor, except for the modest finality of the progestin receptor, which, however, is agonistic in nature (that is, by stimulating these receptors, it gives a progestin message that is not the best in cut).
 If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol… The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. a keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic. Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-metaasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the fewest “hormonal” sides existing at the time. Its ability (common to more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone from which it is structurally derived, it has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong affinity for AR adipose tissue (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).
If we add methylation at the C-17 position to drostanolone, we get [ 2a, 17a-dimethyl-5a-androstan-17b-ol-3-one ], commonly known as methyldrostanolone. Methasterone or Superdrol… The addition of C-17 methylation, in addition to the clearly high oral bioavailability (characteristic of all compounds using this modification), also leads to a decrease in the bond with SHBG and an increase in the stability of C-3. a keto group responsible for the intensity of stimulation of the muscle androgen receptor and partially also non-androgenic. Also this property of methylation of C-17 is, although little known, quite widespread: instead, the effectiveness of the stabilization obtained varies.In this case, the stabilization is remarkable and gives us a product that is orally 400 ANABOLIZERS and 20 ANDROGENS derived from it, is one of the highest when – or equal to that of oxandrolone (20, with a 24/480 ratio), which, however, is slightly more androgenic and more anabolic, at least at moderate doses. Note that both products are tested with reference to methyltestosterone equal to 100/100: this means that compared to injectable testosterone, the SD ratio is about 20/600 with a therapeutic index of 30. However, compared to oxandrolone, the affinity for the superdrol receptor is much higher. selective and typically AR, although there is some predominantly inverse affinity for progestin receptors; hepatic toxicity is also higher given the specific metabolic pathway, mainly outside the liver of oxandrolone. On the other hand, however, the hepatotoxicity of superdrol is usually exaggerated when it comes to doubly toxic AAS due to its double methylation at C-2 and C-17. In fact, methylation at C-2 is associated with irrelevant hepatotoxicity. The reputation of the hepatic killer enjoyed by DM is due, in addition to the double methylation in the name, by an overdose done by relatively many inexperienced users, as it has been marketed since 2005 in the commercial form of prohormones…. In fact, like many of the currently known products, SuperDrol is nothing more than an old molecule used only clinically and never sold before.In fact, methasterone was synthesized in 1956 (like d-bol) to to answer the question about a powerful anti-cancer drug: in this form, it quickly lost popularity and never appeared on the market, officially because of its (declared) excessive hepatotoxicity; in fact, Superdrol-metaasterone was put in the box precisely because it was doing its anticancer job too efficiently: at the time of its initial use, it was actually the strongest AAS and with the fewest “hormonal” sides existing at the time. Its ability (common to more or less all AAS) to prevent and regress tumor development represented a poor commercial operation for the Syntex Corporation (dealing with the anticancer front with other much less effective drugs) and for the organized US hospital industry, organized as a real business that could only flourish based on persistent poor health of the population. Oddly enough, Syntex had no problem commercializing methylated oxymetholone C-17 and is slightly weaker than Superdrol mg x mg with its potency at doses of 500-600 mg per day, which it always killed liver cells. Superdrol is a reduced 5-alpha molecule and is therefore completely resistant to the aromatization of estrogen and, in fact, like mesterolone and drostanolone from which it is structurally derived, it has a certain ability to inhibit aromatase: this, in addition to very low androgenic activity and strong affinity for AR adipose tissue (which exhibits lipolytic activity) makes it a very popular product among high-level athletes… Its use by athletes remains the most logical as it competes with products like trenbolone, which is slightly more anabolic (625) but also much more androgenic (185). Trenbolone also retains the advantage of being it is not toxic to the liver (as long as you avoid exaggerating protein intake while maintaining high fluid intake).