The market for prohormones (PH) and designer steroids (DS) is becoming mainstream among amateur bodybuilders in the United States and will soon become the same for professional ones (this is already for the Olympic, due to the bypass of “doping”).
The reason for this lies in the crossroads between the huge demand for AAS and the ability to circumvent the very strict US laws that treat all AAS as narcotic drugs. The trick is to sell substances outside the FDA’s closed list, which then in turn tries to demonstrate that the substances in question are harmful or converted to testosterone or other banned substances, or are themselves anabolic steroids (but the burden of the test withstands the FDA … and for all things that take time): the ongoing legal battle between supplement traders (thus PH and DS are sold) and the FDA, where new (in fact) AAS are continually invented, launched and withdrawn (sometimes before The FDA is intervening so they can be resold in the future.)
Under these conditions, it is virtually impossible to maintain a complete list of new products with their characteristics, including because these characteristics persist, at least as long as it is never uncertain; This is facilitated not only by the speed of product turnover and the absence of any preventive testing, but also by the habit of distributors to attribute sometimes creative qualities to their substances: checks are almost impossible, and therefore we often come across Semi-inert products that are publicly hyped, or very powerful products that are smuggled are delivered for small supplements for starters, this is delaying FDA checks or simply because at some point the market makes a request.
From the US, a wave of PH & DS has already flooded the UK market (where AAS is legal and these products are sold as supplements without worrying about ostracism from the authorities) … and the first waves that announced the big wave have also arrived in Italy. IN THE NEXT YEARS, KNOWING HOW TO CONTROL PH AND DS, TO CREATE A FUNDAMENTAL TECHNICAL ADVANTAGE also among Italian bodybuilders.
In this situation, we will continue to post the profiles of the main PH and / or DS (they are actually the same thing), but first I think it is more useful to try to provide technical means so that each user can try to understand what they have before him, without attaching too much importance to the various information on the forums in the US and UK (often piloted by distributors). To do this, it is necessary to study the basics of androgenic-steroid chemistry and, in particular, what modifications are used by pharmacological researchers to distinguish new molecules from the main ones:
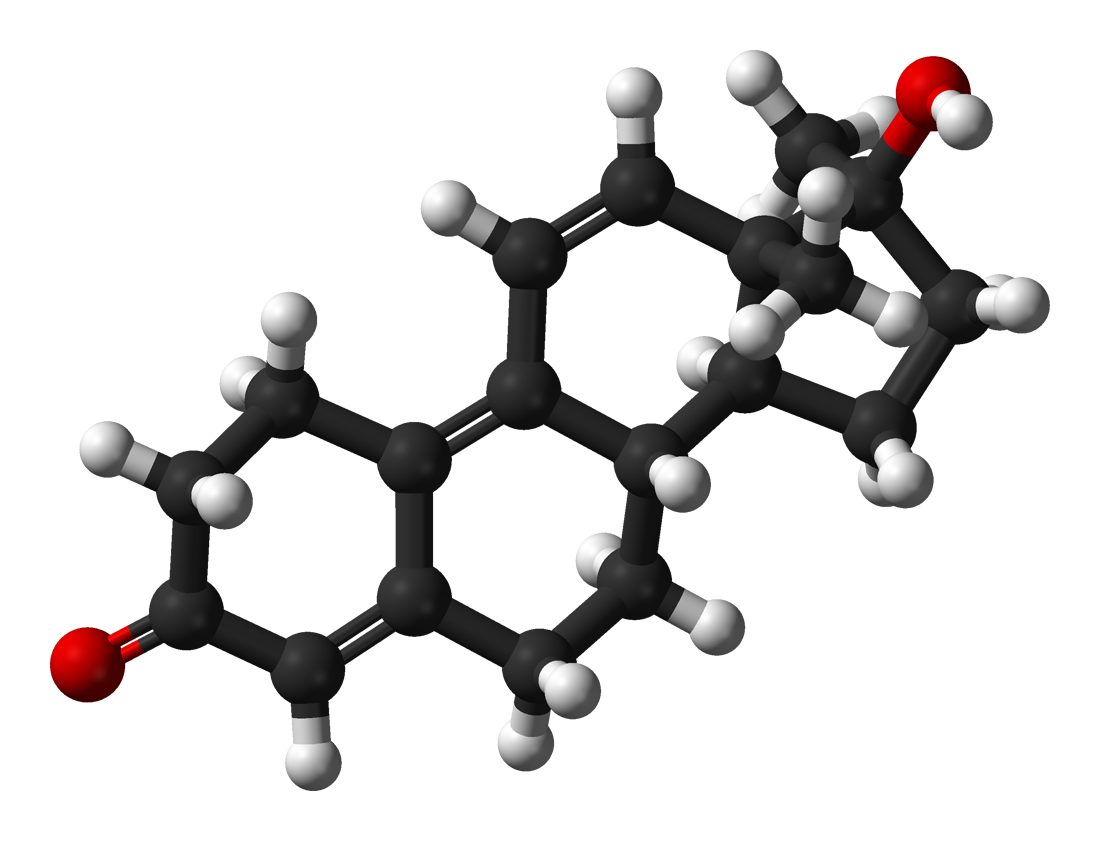
TESTOSTERONE (T: defined as 100 androgenic / 100 anabolic and receptor blends).
The most obvious modifications are those that lead to the formation of other androgenic hormones already present in the human body:
- DEYDROTESTOSTERONE (DHT 100/10, ar). Through interaction with the enzyme 5-alpha reductase on T, it loses the C4-C5 double bond with the addition of two hydrogen atoms to the structure: this modification creates aas with a much stronger affinity than T for androgen receptors, but which are mainly found in the prostate , clitoris, hair follicles, sebaceous glands, while DHT, before it can interact with muscle androgen receptors, is degraded by the enzyme 3-alpha hydrodesosis dehydrogenase (3-alpha-HSD) to an inactive metabolite of androstenediol. In addition, DHT, losing its bond in C4-C5, loses the ability to be attacked by the aromatase enzyme.
- 19-NOR-TESTOSTERONE (NANDROLONE 35/125, ar) differs from T only in the absence of carbon (C) at position 19. Nandrolone owes its low androgenic / anabolic ratio to its potential conversion by the enzyme 5-alpha-reductase to the metabolite dehydronandrolone (DHN), which is poorly bound to androgen receptors.
From the structural basis of these three natural AAS, we can move on to other typical modifications to give special properties to the AAS molecule:
1. The most common is methylation, that is, the addition of methyl to one of the carbon atoms to increase its bioavailability, especially orally, due to the fact that a certain amount of the product passes through the liver barrier without damage. There are different types of methylation, which also have very different effects on other characteristics of the molecule, regardless of bioavailability. however, the 3 most common methylations are as follows:
- The best known is C-17 methylation. The only definite characteristics of this modification are a strong resistance to degradation in the liver and a decrease in the rate of interaction with the aromatase enzyme, which, however, is excessively (in terms of estrogenic activity) compensated by metabolism to 17-alpha-methylestradiol, which is 5 times more similar to the estrogen receptor. than estradiol itself. In addition, a variable decrease in the affinity for SHBG is common, making the molecule more biologically active. The resistance to liver metabolism caused by this type of methylation always causes significant stress in the liver. The potency of the molecule produced varies greatly, especially depending on the stability it brings to the C-3-keto group, vital for resistance to 3-alpha-HSD 5-alpha reduced metabolites and therefore for its effectiveness in muscle tissue. The increase in this aspect can be modest, as in the case of methyltestosterone, or absolutely shocking, as in the case of methyltrenbolone;
- Another common methylation is that at the C-1 position it is beneficial because it provides some resistance to liver metabolism without stress for that organ, but is almost unable to increase the stability of the C-3 keto group, which remains fairly easily attacked by 3- alpha HSD. The affinity for SHBG remains high;
- methylation at position C-2, less effective in increasing resistance to hepatic passage compared to C-17 and C-1, but usually effective in stabilizing the C3-keto group;
- methylation at the C-7 position, which is weak in protecting the molecule from passage through the liver, is more beneficial in reducing the affinity for SHBG and is able to completely inhibit 5-alpha reduction.
2. 17-alpha ethylation, which provides hepatic passage such as C-17 methylation, but with less hepatic stress, but at the expense of a marked increase in progestogen receptors.
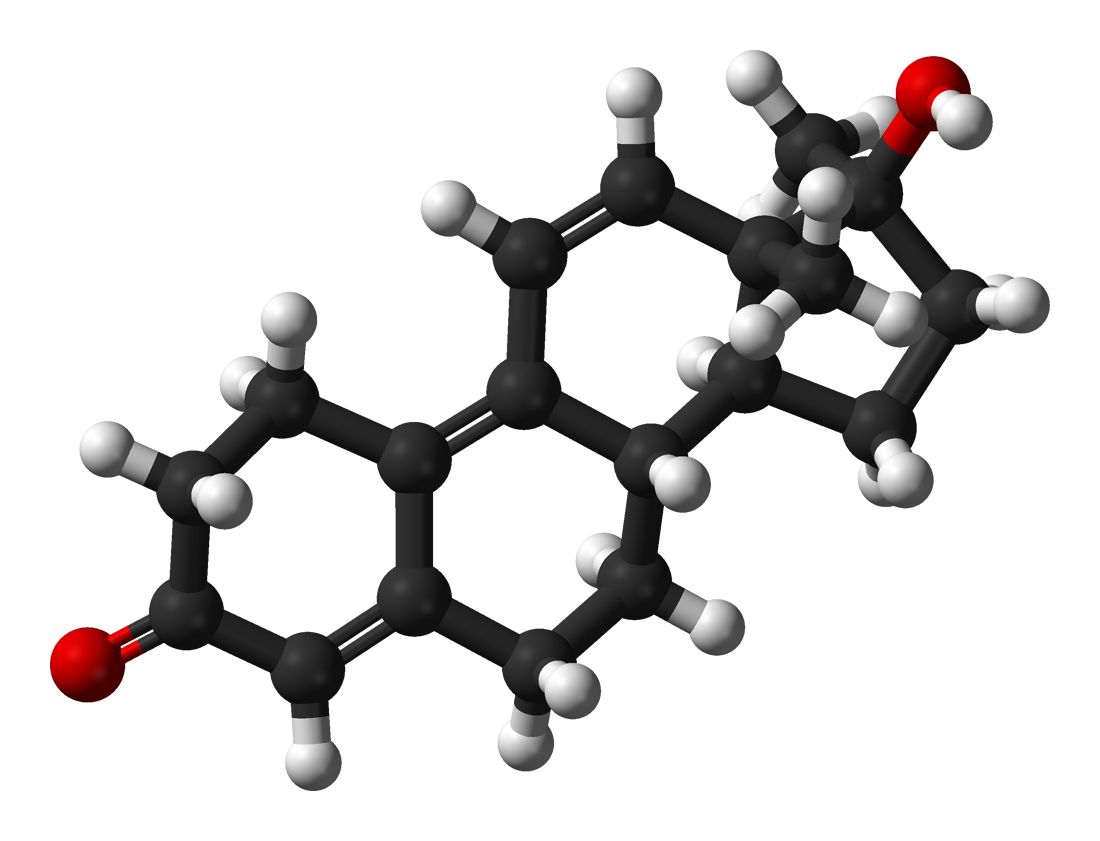
3. Replacement of the keto group at C-3: Instead of looking for solutions to increase the resistance of this group to 3-alpha-HSD, it is sometimes replaced with a configuration that allows it to bind to the androgen receptor, which cannot be attacked by 3-alpha-HSD.
These are the most common AAS modifications, however there are some specific or rarer ones that have proven to be particularly effective and can be found among the PH / DS:
- Replacement in C-2 of an oxygen molecule with a marked resistance to oxidation of the keto group C-3: among traditional AAS, this is a unique modification of oxandrolone.
- Use of hydroxymethyl group at C-2 with strong ketone stabilization at C-3: typical modification of oxandrolone buy
- The introduction of a double bond at C9-C10 inhibits the aromatization of non-5-alpha reduced compounds (e.g. trenbolone).
- Unsaturation in C11-C12 increases the affinity for androgen receptors of nandrolone derivatives (eg trenbolone)
I am sorry for the weight of the exhibition, but I have not found another way to start systematically dealing with this topic. Faced with PH and DS, we cannot do what many of us did at the beginning of our careers, that is, learn to memorize the AAS table: the PH & DS table is not a table, but an amoeba in constant expansion and mutation.