After an article on the allegedly unfounded link between high testosterone levels and post-cancer disease (read “Prostate Cancer: Is Testosterone Justified?”), I propose to dwell on another topic that is partially related to the previous one; or estrogens with their mediating role in the inhibition of the HPTA axis and clomiphene citrate (clomid).
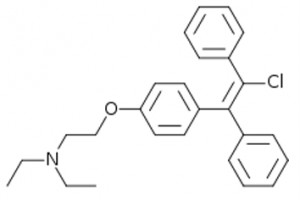 Clomiphene is, as we well know, a well-known antiestrogen (which acts as a weak estrogen on target receptors) belonging to SERM ( S optional E strict R receptor M odulator ), but unlike other antiestrogens, it does not have a strong peripheral receptor effect, but is central at the level of the hypothalamus (and indirectly the pituitary gland). This also explains in part the failure of this molecule, intended as a traditional antiestrogen, to avoid the unpleasant effects of excess estrogen after exogenous consumption of androgens.
Clomiphene is, as we well know, a well-known antiestrogen (which acts as a weak estrogen on target receptors) belonging to SERM ( S optional E strict R receptor M odulator ), but unlike other antiestrogens, it does not have a strong peripheral receptor effect, but is central at the level of the hypothalamus (and indirectly the pituitary gland). This also explains in part the failure of this molecule, intended as a traditional antiestrogen, to avoid the unpleasant effects of excess estrogen after exogenous consumption of androgens.
Clomiphene citrate is used to stimulate ovulation in women (an alternative to the low sides is letrozole [ 1 ] [17] ) and also appears to reduce concentration IGF-1 in plasma by increasing the concentration of IGFBP-1 in women with polycystic ovaries (with a 56.5% decrease in the IGF-1: IGFBP-1 ratio). [ 16 ]
The use of clomiphene in men is not common practice and therefore not much research has been done.
We have come to the pathophysiological mechanism underlying the regulation and maintenance of hormonal homeostasis along the HPTA ( H ypothalamic P ituitary T estes A xis ) what and why it happens when the latter it stops working. [2] [3] [4] [5]
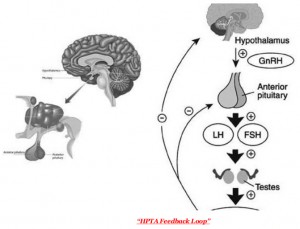
The HPTA axis, also known as the hypothalamus-pituitary-gonad axis, is a system for stimulating / inhibiting hormones produced by the corresponding structures:
- Ipotalamo -> GnRH ( G or n adotropin R elimination H hormone or gonadotropin-releasing hormone)
- Pituitary gland -> LH (luteinizing hormone) and FSH (follicle stimulating hormone)
- Gonadi -> Testosterone , androstenedione, DHEAS, Inibina
The inhibition process occurs as the action of androgens (mediated by estrogens) at the level of the hypothalamus, as well as the pituitary gland, triggers negative feedback, which leads to control or inhibition of stimulation.
When a state of secondary hypogonadism occurs, in which testosterone, LH and FSH values are low (for example, after prolonged use of exogenous androgens after smoking cessation), estrogens are activated and persist for quite a long period of time. inhibiting the release of GnRH, as they interact with the corresponding receptors present in the hypothalamus. Exogenous administration of hCG or chorionic gonadotropin (see article here), which replaces pituitary LH, will lead to increased testosterone levels in the short term [ 20 ] , but, on the one hand, it will not affect the “disinhibition” of the hypothalamus, and on the other hand, it does not affect the control of the flavor that testosterone is exposed to (so you are in a worse position than the original one).
Let’s take a quick look at the main HPTA failure situations in which low testosterone levels are found:
- IPO gonadism IPER gonadotropic
- IPO gonadism IPO gonadotropic
In the first case, we will have high levels of gonadotropin and low levels of testosterone in serum, which indicates possible damage (or desensitization to LH) of Leydig cells, which have a low response to gonadotropic stimulation, whether endogenous (LH) or exogenous (hCG).
In the second case ( IPO gonadism IPO gonadotropic) the problems lie upstream, that is, the pituitary gland and hypothalamus. In this case, serum testosterone levels are low in combination with baseline LH and FSH levels. Low gonadotropin levels may be due to low GnRH levels if the hypothalamus is the problem. So the problems here can be different:
- tumor of the pituitary gland or hypothalamus
- psychoactive substances (eg methamphetamines and derivatives), estrogens (also in the case of an unfavorable T / E ratio [ 18 ] ) and DHT
- congenital deficiency of GnRH or LH, FSH
- other factors that alter the control mechanism of the hypothalamus
It is not easy to understand the origin of the problem and its cause, so let’s make a few attempts. Before I talked about hCG administration, well in case there is IPOgonadotropic hypogonadism, hCG administration is essentially used to assess the response of the gonads and to make sure that at least our symptoms are working. And here is Clomiphene. even so, the answer to our problem cannot be final. It binds (with an antagonistic affinity) to estrogen receptors in the hypothalamus, attenuating the inhibitory feedback signal from estrogen; hence, this leads to an increase in the production of GnRH, which in turn stimulates the production of gonadotropins. If it doesn’t, we still won’t be able to figure out if the malfunctioning problem is really related to the pituitary gland or the hypothalamus. Another “test” is the introduction of GnRH (commercially Kryptocur), which stimulates the release of pituitary gonadotropins.
Therefore, by crossing the results of the clomiphene test with the results of Cryptocuri, one can effectively understand whether damage exists at the level of the hypothalamus or pituitary gland:
- if we do not receive stimulation of the pituitary gland with clomiphene, but we receive it with the help of cryptocuri, this means that there is damage to the hypothalamus (also of a neurological nature, as in the case of MDMA administration [ 28 ] [ 29 ] [ 30 ] [ 31 ] )
- if we don’t get stimulation with cryptocurrency instead, the damage will be caused by the pituitary gland
the nature of the damage, be it the pituitary gland or the hypothalamus, requires additional specialized diagnostic studies.
Now, returning to aspects and situations that are more closely related to the use of androgens, we know that the use of anabolic steroids (especially if they can be aromatized) causes negative feedback on VPTC, therefore it is the most common situation (such as as a result of discontinuation) hypogonadotropic hypogonadism occurs, in which there is no structural damage to the hypothalamus or pituitary gland, but only elements of hypothalamic-pituitary inhibition (high estrogen levels).
Thus, clomiphene in this context is excellent in hypothalamic “disinhibition”; the dose is 100 mg / day, but the duration varies from 1 to 4 weeks. [ 14 ] [ 15 ] The combination of an aromatase inhibitor enhances the effects of clomiphene mainly because it shortens the therapy time. In addition, clomiphene also interacts at the level of the pituitary gland, increasing its sensitivity to GnRH. [ 15 ]
Clomiphene has been shown to be beneficial, effective over time and at low cost, as well as an alternative to substitution therapy, especially in young adults, while maintaining its fertility and in all cases of idiopathic hypogonadotropic hypogonadism. [ 6 ] [ 7 ] [ 8 ] [ 11 ] [ 12 ] [ 13 ] [19]
There is also a proprietary stereoisomer called enclomiphene (androxal), which has more antiestrogenic activity with the advantage of a lower dosage. [ 9 ] [ 10 ]
SIDE EFFECTS
Most of the studies done on the side effects caused by clomiphene use are for women who have been using it for quite long periods to induce ovulation, as previously mentioned. Side effects are of different types:
- Visual impairment : the overall incidence in clinical trials is 1.5% [ 21 ] [ 26 ] [ 27 ]
- Psychotic reactions : In some cases, women treated with clomiphene develop psychotic disorders, but this is a rare occurrence that disappears when therapy is interrupted. In addition, some items were already problematic ones. Symptoms usually appear 2 days after starting therapy [ 22 ] [ 23 ] [ 24 ]
- Transaminity : one case reported in the literature of a Somali woman; this is a very rare side effect of [ 25 ]
CONCLUSIONS
Overall, clomiphene has proven to be an effective, safe and inexpensive product (around € 5, which can be borrowed from the NHS); useful when the goal is to maintain fertility, as in cases of juvenile hypogonadism, and you want to act endogenously by “stimulating” (suppressing the hypothalamus). We also have other drugs today, such as letrozole (Femara), which is very potent as an anti-aromatase, but obviously the cost is increasing compared to plain clomiphene.
Recovery of the HPTA axis is always possible, if there is no structural damage to the organs of which it consists, indeed, recovery can be spontaneous, but with rather long periods of time, such as a weakening of good general state of psychophysical health; hence, faster healing times through disinhibition / stimulation is a fact due to good overall health.
