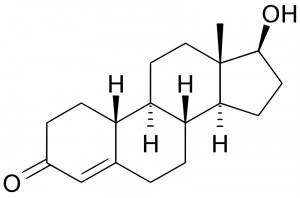
 NANDROLONE estr-4-en-3-one, 17-hydroxy-, (17b) – , basically identical to testosterone, except for the absence of a carbon atom in position 19, hence the name 19-nor-testosterone. Among the natural androgenic-anabolic steroids (testosterone, dehydrotestosterone, androstenediol and nandrolone), nandrolone is the lowest anabolic / androgenic ratio because it is converted by 5-alpha reductase to dihydronadrolone (DHN), an inactive molecule. Even norestradiol, with which it aromatizes 19-N, is less active (about 5 times) than estradiol, and, despite the strong aromatization in the liver, the process at the level of adipose tissue is very slow. 19-N has excellent affinity for AR (androgen) receptors, but it is also able to bind to PR (progestin) receptors with agonist affinity, as well as CR (glucocorticoid) receptors with inverse affinity in some tissues (muscles). agonist in others (eyeball) and mixed in others (connective tissues). The maximum relative dose is 600 mg per week, the absolute 800 – after administration. This product has traditionally been associated well with d-bol for at least 3 reasons:
NANDROLONE estr-4-en-3-one, 17-hydroxy-, (17b) – , basically identical to testosterone, except for the absence of a carbon atom in position 19, hence the name 19-nor-testosterone. Among the natural androgenic-anabolic steroids (testosterone, dehydrotestosterone, androstenediol and nandrolone), nandrolone is the lowest anabolic / androgenic ratio because it is converted by 5-alpha reductase to dihydronadrolone (DHN), an inactive molecule. Even norestradiol, with which it aromatizes 19-N, is less active (about 5 times) than estradiol, and, despite the strong aromatization in the liver, the process at the level of adipose tissue is very slow. 19-N has excellent affinity for AR (androgen) receptors, but it is also able to bind to PR (progestin) receptors with agonist affinity, as well as CR (glucocorticoid) receptors with inverse affinity in some tissues (muscles). agonist in others (eyeball) and mixed in others (connective tissues). The maximum relative dose is 600 mg per week, the absolute 800 – after administration. This product has traditionally been associated well with d-bol for at least 3 reasons:
- because the receptor susceptibility is fully compatible, with d-bol being almost completely non-Ar;
- because the weak norestradiol acts as an estrogen agonist antagonist against the metabolite arising from the aromatization of d-bol, that is, the super-strong methyl estradiol;
- while 19-N stimulates erythropoiesis, d-bol is one of the few donkeys that suppresses it.
Unfortunately, 19-N has a strong antigonadal effect: it can completely suppress the production of gonadotropins only with 200 mg of esterified product per week. This is probably largely due to the affinity of the nandrolone agonist for the PG receptors and the resulting stimulation of prolactin: therefore, it is recommended to take antiprogestogens in combination with deca or other constructors or ph derived from nadrolone.
The androgenic / anabolic ratio in nadroloene is 37/125.
The true proprons (PH) of nadrolone, that is, the compounds that convert to nadrolone, are:
- A compound commonly known as 19-norandrostenediol or 19-norandrostenedione (19-norandrost-4-ene-3,17-dione or 19-norandrost-4-ene-3,17-diol or 4-estrene -3,17-dione): This is the simple addition of ketone diol to nadrolone, which usually results in a conversion of about 80%. The weak point of this PH is its low oral bioavailability
- DECASTERONE (3b-enanthoxy-19-nor-androst-4-en-17-one), which is the precursor of two-pass nandrolone: 3b-enanthoxy-19-nor-androst-4-ene -17-one -> 19 -norandrostenediol -> 19-nor-testosterone. The conversion to nandrolone is likely to be lower than that of 19-norandrostenediol, but oral bioavailability is somewhat improved by binding to the enanthate ester, the lipophilic properties of which should allow some use of the lymphatic system for assimilation, bypassing the liver to a small extent.
The Designer Steroid (DS) series is currently sourced from nandrolone, currently marketed as ph and therefore as dietary supplements. We exclude trenbolone and its closest derivatives, which we analyzed in the previous post.
Among DS nandrolone we have:
- NORETHANDROLON (19-Norpregn-4-en-3-one, 17-hydroxy-, (17α) – known as NYLEVAR, was the first oral drug to be marketed in the US It is simply nandrolone with an added ethyl group of 17 alpha, which makes the product able to cross the hepatic barrier as efficiently as methylated alpha, is also almost equally toxic.This addition increases its affinity for progestin and decreases it for androgen receptors, converting norethandrol into a mixed type aase. prolactin (the release of which is stimulated by the activation of progestogen receptors) of related compounds is very important, which contributes to the increase in water retention caused by prolactin itself.The most striking effect of this product, in addition to its strong antigonadotropic effect, is a very rapid amnetting of a dirty mass, mainly due to water retention: however, let’s not forget that protein synthesis is stimulated by hydration. muscle cell ion. a is 35 androgens / 150 anabolic.
- ETHYLESTRNOLO (19-nor-17alpha-pregn-4-an-17-ol or 19-nor-17alpha-ethyl-19-norandrost – 4-en -17b-ol] 0rgabolin, maxibolin) is also an old AAS, similar to Nilevar, except for the lack of a ketone at C-3, fundamental for the stability of binding to the androgen receptor, which makes it completely non-AR EET; however, the androgenic / anabolic EES ratio is 20/400. There are two hypotheses explaining the high anabolic activity of the product, despite the absence of the 3-keto group: I. EES acts completely non-ar; II. s I am testing the formation of ketone in C-3, which converts EES to norethandrolone.
The second hypothesis seems unlikely, since ethylestrenol has very different characteristics from norethandrolone: primarily less progestogenic, less androgenic and more anabolic.
- NORBOLETON (13-ethyl-17-hydroxy-18,19-dinor-17-pregn-4-en-20-yn-3-one or, more simply, 13b-ethyl-nor-androstenedione) or GENABOL. The main difference from norentandrolone (nilevar) is the inclusion of an ethyl group in C-18 instead of the classic methyl group in alpha-C-17, this has various effects: I. makes the molecule slightly less hepatotoxic; II. 3rd keto group is strongly destabilized when passing through the liver with very low activity against AR-receptors; III. increases the already high affinity for the Nilevar receptor for progesterone receptors (PR), but in a reverse, not agonistic manner.
In fact, this also applies to the old AAS, created in 1964, whose characteristics we know very well: very low aromatization rate, lower than that of a deca, and with an anabolic androgenic ratio of 17/350. It is a steroid with a progestin structure, but unlike nandrolone and norethandrolone, trenbolone and other nandrolone derivatives, its affinity for progestin receptors is not agonistic, but the opposite: this means that the molecule counteracts the effects of the progestin, such as increased prolactin levels and increased aromatase activity … In fact, it has a structure similar to the RU-486 tablet. Genabol also has an inverse effect on cortisol receptors, and this increases the anabolic effect higher than that of nadrolone, despite the almost non-existent affinity for AR. Since this is actually an old AAS created in 1964, we are well aware of its characteristics: very low aromatization rate, lower than that of a deca, and with an anabolic androgenic ratio of 17/350. Precisely because it is old and long out of use, this AAS found widespread use among Olympic athletes until it was identified in 2001. However, the instability of the 3-keto group limits its use in terms of the maximum effective dose (similar to D-bol, which, however, can also use estrogen receptors). In fact, a test carried out on sedentary patients in a postoperative state showed an increase in their effect only up to 7.5 mg / day: probably at 100 kg / day this figure should be increased to 20-30 mg / day.

The most famous PH of norboleton is MAX LMG. METOXYGONADIENE (LMG) is 13-ethyl-3-methoxy-gone-2,5 (10) -diene-17-one17-dimethyl-5a-androst-3-one-17b-oi. It has no methylation at C-17 alpha, but an ethyl group at C-18 has been added to increase its oral activity, making it slightly less hepatotoxic. In the stomach, the methoxy group in C-3 is quickly removed, and the double bond in C is lost in ring A. But at this moment, diacetic acid is formed and the metabolite 13b-ethyl-nor-androstenedione, that is, norboletone, is formed