Floxymesterone or fluoxymesterone [ 9-fluoro-11,17-dihydroxy-10,13,17-trimethyl-1,2,6,7,8,11,12,14 , 15,16-decahydrocyclopenta [a] phenanthrene-3-one ], also known by the trade name Halotestin , is a testosterone-derived anabolic steroid.
Its history relates to Upjohn (or The Upjohn Company), a public company based in Kalamazoo, Michigan, USA, founded in 1886 by Dr. William E. Upjohn and his three brothers. It was one of the largest innovative ethical drug manufacturers in the United States. The Upjohn group of researchers began a series of studies on the potential of anabolic steroids after observing large clinical trials for “deponortoneedone” (Upjohn’s product containing injectable nandrolone) to develop and test a number of new molecules.
One of the most significant discoveries of the Upjohn team was made in 1950, when they discovered a microbiological fermentation technique to introduce an oxygen atom into the 11th carbon of the [1] steroid. This not only allowed the widespread adoption of corticosteroid compounds such as cortisone, but also paved the way for the creation of fluoxymesterone.
Results from experiments on rats treated with some of Upjohn’s 11-oxygenated steroids can be seen in the following [2] table:
As can be seen from the table, all 11-oxygenated steroids were significantly more effective than their non-oxygenated counterparts.
9α-halogenated corticosteroid derivatives were described by Fried and Sabo in 1953, and there was an increase in glucocorticoid and anti-inflammatory activity [3] . With this in mind, Lyster et al.. in Upjohn they synthesized and tested a number of testosterone derivatives 11β-hydroxylated and 9α-halogenated [4] .
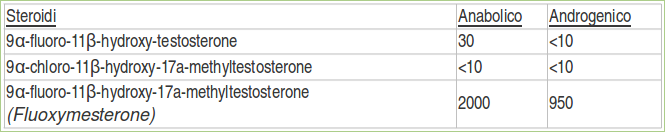
They found that suppression of the activity of the 9-chlorine group and substitution of 9α-fluorine in the steroid led to a significant increase in anabolic and androgenic activity orally in rats [4] :
“11β-Hydroxymethyltestosterone is 0.9 times more androgenic and 3 times more anabolic than methyltestosterone. 9-Fluoro-11β-hydroxymethyltestosterone is 9.5 times more androgenic and 20 times more anabolic than methyltestosterone “
Compound 9α- influenza gold 11β-hydra hydroxy 17α- me tiltesto sterone was subsequently named Fluoxymesterone .
Upjohn researchers classified the anabolic strength: androgen fluoxymesterone administered orally in 2000: 950 compared to methyltestosterone. [4]
Subsequently, the data were presented by Upjohn in 1963 by researchers Kincl and Dorfman of Syntex, who confirmed the data on levator ani, seminal vesicles and ventral prostate by 1745%, 757% and 118%, respectively (the latter two as androgenic indices). [5]
![Il rapporto A:A dello Stanozololo non appare così favorevole dalle analisi della Upjohn del 1965.[8]](https://muscle-pharma24.com/wp-content/uploads/2020/10/fluoxymesterone-halotestin_5f843f6ee6f0a-2.png)
Ratio of stanozolol A: not so favorable in Upjohn’s 1965 analysis. [8]
On a return visit in 1963, the anabolic value of 380 (measured as nitrogen retention) and androgen 140 (measured with the size of the ventral prostate) are always relative to the methyltestosterone of bolsterone and fluoxymesterone, they were found to be “significantly lower than those reported for some 19-nor steroids and some heterocyclic steroids of current clinical interest” [6]
Of course, their conclusions could be somewhat “softened” by their commercial stance; The Sterling-Winthrop researchers were responsible for the creation of the steroid Stanozolol (Winstrol). [7]
Molecular structure of fluoxymesterone
Fluoxymesterone is structurally classified as a halogenated testosterone derivative. The added halogen group is the fluorine group at C-9, which enhances its androgenic activity, making the molecule the best substrate for 5α-reduction to form highly androgenic metabolites. Stanozolol-Injektion preis Although a change in C-9 is an abnormality in the androgen world, it is relatively common among synthetic glucocorticoids; like
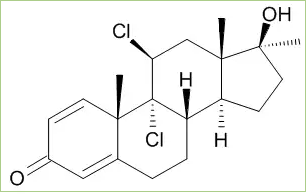
9α, 11β-dichloro-17β-hydroxy-17α-methyandrosta-1,4-dien-3-one (dichlorodianabol) in dexamethasone, betamethasone, fludrocortisone and triamcinolone. There are actually some compounds that are substituted in C-9 with other halogens. For example, beclomethasone has the function of 9α-chlorine. Schering has tested some 9α, 11β-halogenated compounds, but with little success, even though chlorinated derivatives from Dianabol have proven promising as anabolic, with a ratio of 300: 60 compared to oral methyltestosterone. [9]
It is not entirely clear why the 9α-fluorine fraction gives orally administered fluoxymesterone high anabolic / androgenic activity, but the fluorine atom could potentially alter the pharmacokinetics and pharmacodynamics of the compound in several ways
“1. Fluorine, due to its small steric size, resembles biologically active hydrogen compared to a steric environment due to the regions of protein receptors;
2. Fluorine, the most electronegative element, modifies electronic effects;
3. Fluorine, when bound to carbon, has a carbon-fluorine bond energy of 107 kcal / mol, increasing oxidizability, temperature, and metabolic stability; and
4. Perhaps more importantly, carbon-fluorine bonds significantly increase lipid solubility and hence improve bioabsorption and biotransport rates. “ [10]
According to the first property mentioned above, the fluorine atom has a van der Waals radius very similar to the hydrogen atom it replaces, the standard hydrogen radius is about 1.1-1.2 Å. and for fluorine, about 1.35-1.47 Å. [11] [12] [13]
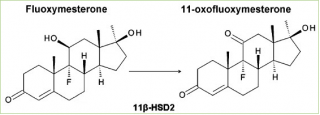
In addition to its unusual 9α-fluoro group, fluoxymesterone also has an 11β-hydroxyl group, which is common in many active corticosteroids. A recent study has shown that due to the presence of the 11β-hydroxyl group, fluoxymesterone interferes with the metabolism of corticosteroids. Cortisol is converted to inactive cortisone by the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD-2). Since the mineralocorticoid (MR) receptor can be activated by both mineralocorticoids and glucocorticoids, this enzyme is expressed in sensitive mineralocorticoid tissues to prevent MR activation by cortisol. Fluoxymesterone has been found to be a potent competitive inhibitor of human 11β-HSD-2. [14] This inhibition can cause cortisol-induced MR activation, which can lead to sodium retention and potassium excretion, causing water retention and hypertension.
Following clinical follow-up of fluoxymesterone some options: 16α-methylated compounds from Merck were synthesized, although they were disappointing in comparison. [15]
11-keto, an analogue of fluoxymesterone, is slightly more anabolic (22 versus 20) and less androgenic (8.5 versus 9.5) than the latter. [16]
Fluoxymesterone metabolism
Despite modifications to 9α-fluorine and 11β-hydroxyl, fluoxymesterone undergoes both 5α-reduction and 5β-reduction in humans, reduced 3-keto metabolites have also been found in people. [17] [18]
Although 9α-fluoro-testosterone aromatizes about half of testosterone, the 11β-hydroxyl group of fluoxymesterone prevents it from aromatization as well as connection affinity with SHBG. [19] [20]
“The presence of axial substituents at position C-11 interferes with aromatization of ring A” [19]
As with all 17α-alkyl-17b-hydroxysteroids, 17-keto metabolites of fluoxymesterone are not possible. It has been reported that 17-epimerization of fluoxymesterone is a common (albeit minor) metabolic pathway among 17α-methyl-17β-hydroxysteroids. [21]
According to a recent study, the presence of several metabolites of hydroxylated, dihydroxylated, 11,12 and 13,14 unsaturated and 11-keto (after administration) in humans has also been identified. [17]
It is also interesting to note that fluoxymesterone is metabolized to its corresponding analogue 11-keto (11-oxo-fluoxymesterone) by human 11β-HSD-2 (although not widely), as demonstrated by modern experiment.
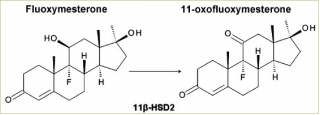
“Biological data indicate that fluoxymesterone is a substrate for 11β-HSD-2, but with a lower conversion rate than cortisol.” [14]
The final difference compared to testosterone is 17-alpha methylation, which apparently makes it toxic to the liver (some evidence suggests severe toxicity). is carried out by methyltestosterone, although it cannot be aromatized and occurs most often at the testicular level. In the 20 mg / day range, it does not appear to have a significant effect on FSH and LH, but it already affects circulating testosterone.
Fluoxymesterone is 100% bioavailable due to methylation at position 17α, which inhibits liver metabolism by 17β enzymatic oxidation -hydroxyl, which allows the molecule to be absorbed into the bloodstream.
Like many other methylated C-17 steroids, fluoxymesterone has a weak affinity for AR receptors, but its actions are mediated by the androgen receptor, largely due to its long half-life in plasma which is about 9.2 hours. [22]
Clinical Uses of Fluoxymesterone
Fluoxymesterone is used in the medical field to treat male hypogonadism, delayed puberty in men and in the treatment of breast cancer in women. The antitumor activity of fluoxymesterone appears to be related to the competitive decrease or inhibition of prolactin or estrogen receptors or their inhibited production.
Sports Training Applications
Given the aforementioned characteristics, fluoxymesterone has been used for a long time in the cultural field and in powerlifting. At BodyBuilding, this AAS was widely used 4-6 weeks before the race, in conjunction with an adequate diet for its ability to improve muscle consistency and increase mental alertness (aggressiveness, resistance to pain and fatigue). ). Since this drug is non-aromatized and rarely causes water retention, it works well enough for this purpose. Powerlifters, on the other hand, interested in the strong androgenic potential of fluoxymesterone, use this molecule solely to increase strength and therefore exertion.
The advent of aromatase inhibitors and the greater availability of trenbolone have removed some of the benefits practically stemming from the use of this product (especially in pre- race), but due to its low affinity for androgen receptors (AR), it still retains some popularity in pre-race and in strength cycles due to products that are distinctly similar for androgen receptors, such as trenbolone.
With the spread of the “Crossfit” phenomenon, Fluoxymesterone has found new sports applications in addition to the first categories mentioned (Bodybuilder and Powerlifter). In fact, this AAS is ideal for this discipline, providing the athlete with great strength, concentration and mental and physical endurance. A stack of fluoxymesterone, trenbolone and GW1516 has great potential for experienced athletes. Dosages of fluoxymesterone used on average by male athletes are 20 to 40 mg / day for a period of 3 to 6 weeks; The dosage is divided into 2-3 equal allowances per day. Taking into account its side effects on the liver, its consumption should never exceed just 6 weeks.
Its availability on the black market is not so great, as well as the presence of products containing or not containing other molecules and excreted as fluoxymesterone. this is not uncommon.
Side effects that fluoxymesterone can cause include headache, nervousness, anxiety, oligospermia, sexual hyperactivity, testicular atrophy, liver cancer, changes in blood lipids and alopecia.
Conclusion
In conclusion, I would like to emphasize the fact that this molecule is not suitable for beginners (with less than 2 cycles performed well) and for sensitive subjects on a psychological level. Never combine Fluoxymesterone and Trenbolone until you have verified the individual tolerance of each of these two molecules. Do-it-yourself as usual is strongly discouraged.
For agonists, it is important to know that anti-doping tests detect fluoxymesterone in 2 months