This is a question that should not be asked in the abstract, but compared to the molecule’s oral bioavailability, toxicity, potency, and price versus the “traditional” version, if any.
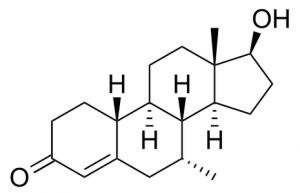 One of the most promising steroid designers used in recent years is 7-alpha-19-nor-androst-4-en-3-one, 17b-ol trestolone strong> (MENT ). As a 19-nor, i.e., a nandrolone derivative, it closely resembles the latter molecule, apart from the presence of a 7-alpha methyl bond, which prevents the 5 alpha molecule from being rebuilt in addition to enhancing the androgenic bond. Although nandrolone is a fairly potent hormone, it is the lowering of 5 alpha that weakens it. Another nandrolone buy derivative without the ability to lower 5 alpha levels is trenbolone. But due to the C9-C10 double bond, this does not lead to aromatization, as Trestolone does, which is not equipped with this double bond. In particular, Trestolone is aromatized with the particularly potent 7-alpha-methyl-estradiol, which enhances Ment’s characteristics as an ideal high-volume steroid: greater estrogen receptor proliferation, better glucose utilization, increased secretion of GH and IGF-1. The activity of this estrogenic activity may be the reason for the greater strength of Ment (960 anabolic / 165 androgens) compared to trenbolone (in turn 600/200 on testosterone), as well as its antigonadal activity, which is 20% higher than that of testosterone. Ment is simply methylated at the 7-alpha position, which, on the one hand, provides good resistance to the passage of the liver (about 50%) with relatively low toxicity to the liver, on the other hand, it cancels the affinity for the sex hormone binding protein. (SBHG), which further increases its power.
One of the most promising steroid designers used in recent years is 7-alpha-19-nor-androst-4-en-3-one, 17b-ol trestolone strong> (MENT ). As a 19-nor, i.e., a nandrolone derivative, it closely resembles the latter molecule, apart from the presence of a 7-alpha methyl bond, which prevents the 5 alpha molecule from being rebuilt in addition to enhancing the androgenic bond. Although nandrolone is a fairly potent hormone, it is the lowering of 5 alpha that weakens it. Another nandrolone buy derivative without the ability to lower 5 alpha levels is trenbolone. But due to the C9-C10 double bond, this does not lead to aromatization, as Trestolone does, which is not equipped with this double bond. In particular, Trestolone is aromatized with the particularly potent 7-alpha-methyl-estradiol, which enhances Ment’s characteristics as an ideal high-volume steroid: greater estrogen receptor proliferation, better glucose utilization, increased secretion of GH and IGF-1. The activity of this estrogenic activity may be the reason for the greater strength of Ment (960 anabolic / 165 androgens) compared to trenbolone (in turn 600/200 on testosterone), as well as its antigonadal activity, which is 20% higher than that of testosterone. Ment is simply methylated at the 7-alpha position, which, on the one hand, provides good resistance to the passage of the liver (about 50%) with relatively low toxicity to the liver, on the other hand, it cancels the affinity for the sex hormone binding protein. (SBHG), which further increases its power.
In particular, Ment’s hepatotoxicity (measured when liver enzymes are elevated beyond the normal range) is zero at 50 mg per day, while at a dose of 100 mg, a dose of semi-toxicity is observed (i.e. half of the subjects who take it begin to report changes liver enzymes). in addition to the norm). Ment’s affinity for progesterone receptors (and therefore a tendency to stimulate prolactin) is similar to that of trenbolone. In all respects, ment looks like an ideal compound for free-flowing and guarantees especially effective effects with other anabolic steroids “for bulk” and without AR affinity, such as D-bol and Oximetolone. The introduction to the sports market of this steroid constructor (which has been successfully developed for thousands of uses, from contraception to the treatment of sarcopenia) brought with it a revival of a very old DS used to identify bitches’ inspiration and increase the aggressiveness of athletes: let’s talk about MIBOLERONE (drop test). that is, the dimethylated version of Ment C-17: 7-alpha, 17-alpha-dimethyl-19Nor -androst-4-en-3-one, 17b-ol , much more hepatotoxic than Ment, thanks to double methylation C-17; however, this does not appear to significantly stabilize the androgenic bond of the 3-keto group, instead a stability that appears to be insufficiently strengthened to compete with the combined action of trestolone with its 7-alpha-methyl-estradiol. In fact, the double methylation at C-17 of mibolerone is also a brake on aromatization, which remains very low in this compound: confirming the importance of estrogenic action as a support for androgenic-anabolic action, we have the less potent Ment compound, being Mibolerone “only” 250 anabolic / 590 androgenic.

Recently 7a-methyl-estra-4-en-3,17-dione is a new prohormone MENTABOLAN, which is often referenced in forums. like ment-dione: it is actually a trestolone with the addition of 17 ketone, where nandrolone has a 17b-hydroxyl function. In the body, ketone C-17 will be hydrolyzed by 17-beta-hydroxysteroid dehydrogenase type 17 (17b-HSD1) in the active compound Trestolone (ment). The conversion of this product to trestolone is about 80%, while the oral bioavailability of trestolen and about half of it intramuscularly.

